3D Bioprinting Tools Provide New Avenues for Medical Research
R&D
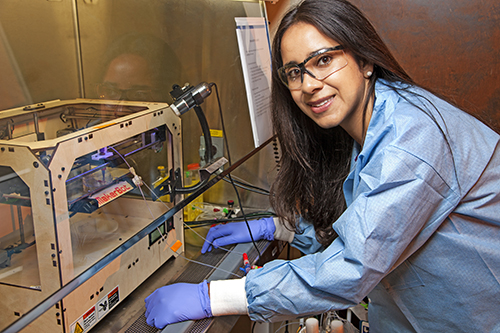 |
| Lawrence Livermore National Laboratory researcher
Monica Moya with the MakerBot she used for printing blood vessels. Photo: LLNL |
3D printing is one of the hottest forms of additive manufacturing techniques due to its ability to quickly and efficiently create custom designs in a wide range of materials. It became apparent early on in the development of these techniques that this methodology would be appropriate for medical prosthetics. 3D-printed prosthetics can be customized to the dimensions of specific individuals and the specialized requirements for each potential patient. The natural evolution of this is the 3D printing of biological constructs—and even artificial organs that can be implanted—rather than utilizing the limited availability of donor-sourced medical transplants.
Numerous conventionally 3D-printed inert prosthetic components have been successfully created and implanted over the past ten years, mostly for medical structural support applications. These include vertebrae, mandibles, thoracic cages and cartilage-based materials such as ears, noses and trachea.
Alternatively, 3D bioprinting involves the use of cells to create three-dimensional living constructs—layer by layer. 3D bioprinting is still in the early stages of R&D with numerous techniques, patents and even the equipment still being developed. The basic 3D bioprinting process employs a 3D printer-based deposition of bioinks to create biologically applicable structures layer by layer, resulting in a functioning living tissue or organ.
There are numerous research applications for these 3D bioprinting tools and processes, including the development of advanced bioinks, biosensors, medical constructs, pills, implants, advanced prosthetics, food and animal product testing, tissue and organ generation and dental repairs/replacements. According to a recent report by market research firm, Grand View Research, the 3D bioprinting market (including bioprinter devices) was estimated at $487 million in 2014 and forecast to grow to $1.8 billion by 2022 for a CAGR of 18% over eight years.
The Tools
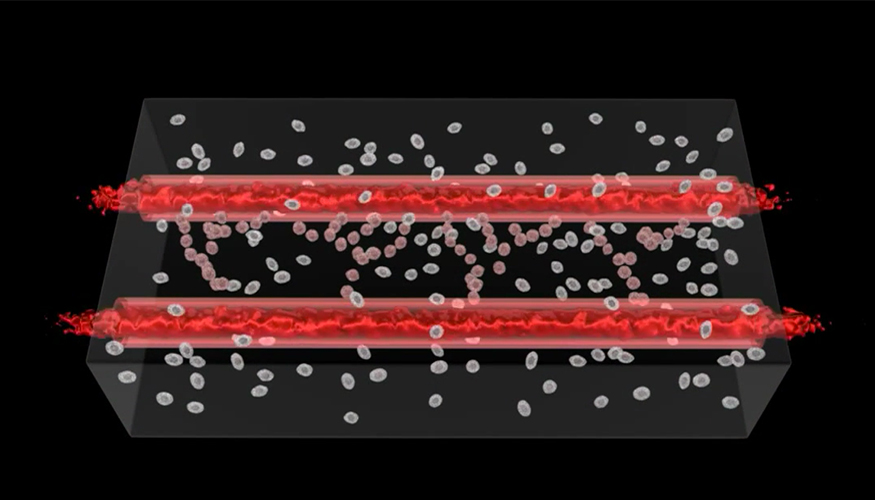 |
| Researchers at Lawrence Livermore National Laboratory
say the precision and 3D structures made possible through bioprinting are enabling them to more effectively reproduce human physiology outside of the body, which will eventually lead to a better representation of each tissue system that makes up the human body. Photo: LLNL |
During the past several years, research groups have developed a number of 3D bioprinters for various life science research investigations. Organovo, San Diego, is the largest of these—it was the first company to commercialize the 3D bioprinting of human tissue with the first cellular blood vessel in 2010. Their NovoGen MMX 3D bioprinter employs syringe-based extrusion techniques to produce tissues with 20 or more cell layers consisting of human cells and nutrient-containing microgels. Organovo produces human liver tissues with this technology that it then provides to pharmaceutical companies for preclinical testing and drug discovery research (the company does not market its in-house developed 3D bioprinter). Organovo also is working with L'Oreal to develop 3D printed skin. They're also working to develop a 3D process for printing kidney tissues similar to their liver printing process.
Tokyo-based Cyfuse Biomedical is developing a 3D bioprinter, Regenova, with a methodology termed Kenzan which uses cellular spheroids containing thousands of cells that are organized and cultured which then leads to self-organization and eventually human tissues. They're working on a number of tissue applications including blood vessels (vasculature), digestive and urinary organs, cartilage, and liver tissues.
Researchers at Aspect Biosystems, Vancouver, BC, Canada, have developed a Lab-on-a-Printer technology which can fabricate 3D printed tissues utilizing different cells, biomaterials and growth factors. This allows them to create macro-scale 3D structures that incorporate micro-level details for programming and creating architecturally and functionally accurate human tissues. The company also offers contract testing services for 3D human tissue models.
Similarly, Ourobotics, Cork, Ireland, has created the Ourobotics Revolution 3D bioprinter that can print up to 10 materials in the same bioprinted structure, which can theoretically be expanded to any number of materials. The relatively low-cost device currently prints a variety of gel-like materials, including collagen, gelatin, alginates and chitosan and includes a heated enclosure that allows researchers to incubate living cells.
Philadelphia, Pa.-based BioBots has also created a low-cost 3D bioprinter, along with specially formulated bio-inks. Their 3D bioprinter utilizes cartridge technologies similar to those in inkjet printers, which can build multi-layered tissues with up to 100 mm-resolution. Following printing, the tissue is cured with a special blue light that does not damage the living cells. BioBot bioprinter cartridges contain three powders that are mixed with a fourth binding factor powder and the living cells. The mixture is then pushed through a syringe onto a tissue culture dish where the blue light is applied.
Traditional 3D printer company, Rokit, South Korea, recently announced that it will expand its product line to include an in situ 3D bioprinter. Rokit is collaborating with the Korean Institute of Science and Technology, Seoul National Univ., Bundang Hospital, Hanyang Univ. and the Korea Institute of Machinery and Materials on this development which is targeted at printing human skin for burn victims, dermatological diseases and cosmetic surgeries. The company expects to complete development by 2018.
Other companies developing 3D bioprinters and associated equipment, processes and technologies include:
- 3D Bioprinting Solutions, Moscow, Russia—3D printing systems utilizing stem cells taken from a patient which are mixed with hydrogels before being printed. The hydrogels dissolve leaving only the living cells.
- Advanced Solutions, Louisville, KY—The fully-equipped BioAssemblyBot comes with Tissue Structure Information Modeling software that allows researchers to design, visualize and fabricate complex tissue structures.
- Bio3D, Singapore—SYN^ can print whole cells, bacteria, proteins, biogels, polymers, food materials and more. Their Explorer system is an entry level bioprinter for beginners and educators.
- Cellink, Palo Alto, CA—The low-cost INKREDIBLE 3D bioprinter comes with dual print heads.
- EnvisionTEC, Dearborn, MI—Their 3D Bioplotter System's syringe-based extrusion process utilizes a wide range of materials including soft hydrogels to build 3D bio-scaffolds/
- GeSim, Mannsdorf, Germany—The bioscaffolder is a modular 3D bioprinting platform targeted at multiple material bioscaffolds with defined linear structures and the printing of live cells either embedded in the scaffold material or seeded by piezoelectric micro-dispensers.
- Modern Meadow, Brooklyn, NY—3D printing of meat foods and leather products.
- Nano3D Biosciences, Houston, TX – Printing of 3D breast cancer tissues for biological research.
- Regemat 3D, Grenada, Spain—Initial 3D bioprinters focused on treating cartilage degeneration due to the overall large size of the potential market. Systems replicate native form and function of tissues.
- RegenHU, Villaz Saint Pierre, Switzerland—Their BioFactory and 3DDiscovery utilize proprietary bioinks and software making them suppliers of one of the most comprehensive bioprinting systems.
- Shenzhen Technology, Guangdong, China—Their Maipu Regenerative Medical Technology's MedPrin utilizes 3D-printed dura mater (the membrane encasing the brain) for use in brain surgeries.
- Sichuan Revotek, Chengdu, China—Created 3D blood vessel bioprinter.
- TeVido Biodevices, Austin, TX – Printing of skin tissues for breast reconstructive surgery.
Post-3D printing
A common 3D printing technology employs the use of 3D printing equipment to create a life science-based scaffold onto which is embedded live cells that are cultured to create living tissues. Research engineer Monica Moya at the Dept. of Energy's Lawrence Livermore National Laboratory (LLNL), Livermore, CA, is involved in research investigating the post-3D bioprinting processes of building living and functioning human tissues.
(Editor's Note: Some of the following was taken from an exclusive live interview with R&D with Monica Moya and some was excerpted from an extensive [more than 200 comments] Bioprinting AMA [Ask Me Anything] reddit session on December 3, 2015.)
3D bioprinting of human organs is a very complex endeavor consisting of a lot of different types of cells, connections, materials supply/routing/removal, compatibility concerns and growth controls. “You cannot just grab a few billion muscle cells and make a heart,” says Moya. “You also need modified conductive muscle cells, a blood supply, nervous system supply, connective tissues and more. And you need all of them in the right place. And you need a lot of them.”
Stem cells are good for these applications, according to Moya. “However, there are still efficiency challenges. To keep them proliferating, you have to keep stem cells in an undifferentiated (immature) state, which takes a specific set of factors, then you need to induce them to differentiate and supply the appropriate growth medium,” she explains. Other concerns with stem cells include purity, yield, physical culturing, scaffold size, growth accommodations and more.
With all of the limitations inherent in culturing 3D printed organ, Moya decided to focus her research on one of the basic components—the development of blood vessels, which is funded by in-house research programs. “Without blood vessels, new 3D printed parts and implants cannot integrate and grow successfully into the human body,” says Moya. She is also involved in the development, with co-LLNL researcher Elizabeth Wheeler, of an in vitro Chip-based Human Investigational Platform (iCHIP) which attempts to create the central and peripheral nervous systems, the blood-brain barrier and the heart—basically a brain-on-a-chip system.
“In the long-term future, tissue on chip systems could replace human subjects (for evaluation of new therapeutical products and processes) once they've been fully validated,” says Moya. “But that is a long ways off.”
For her 3D bioprinting, Moya uses a simple commercially available MakerBot 3D printer that's easy to use and easy to modify. She likes this system because of its small size and ease of being placed in a biosafety cabinet to ensure the safety of her living cells. The blood vessels she creates are approximately 800 microns in diameter—large enough to stick a hypodermic needle for research purposes. Smaller capillaries are common in living humans, and they are possible to be created with 3D bioprinters, but that size of soft tissues are inappropriate for monitoring with needles.
To create her “synthetic” blood vessels, Moya prints the cells directly onto the scaffold. “Even in the blood vessels, we print support cells as part of the tubes (scaffold),” she says. “We then seed endothelial cells inside the tubes to create the endothelium that's normally found in cells. We spend a good amount of research characterizing how our materials gel as they are extruded because we need to understand what forces our cells feel and to make sure that we keep them alive.”
Another concern with 3D bioprinting research is that the structures you're working with are 3D objects and 2D-based culturing methodologies, like traditional Petri dish cultures, and are no longer appropriate for creating a growth environment. “3D bioprinted blood vessels also need to be cultured in a dynamic way—media has to be pumped through the vessels for them to grow,” says Moya. “The media flowing through the vessel also needs a precise pressure gradient that's maintainable, controllable and measurable.”
Future realities
While the complexities of creating functioning 3D bioprinted organs still have many hurdles to overcome, there are significant incentives for continuing to push the limits of today's research. Not the least of these is that organ donor rates continue to fall, with an increasing number of patients on wait lists for these organs. Accelerated methodologies for testing the drug discovery and development of new chemical and biological compounds is also encouraging the development of 3D printing technologies.
Building customized organs with 3D bioprinting technologies can solve the complexity issues by co-engineering the future. “We're leveraging the body's ability for self-directed growth with 3D printed organs.” says Moya.
Significant advances are also being made in simple organs, such as skin disorders/injuries and thyroid glands. Russia's 3D Printing Solutions, for example, has successfully 3D printed a thyroid gland for a mouse and transplanted it into living mice with restored function after three months. Organovo has also supplied bioprinted human skin for use in testing of cosmetic products to L'Oreal—processes that previously were performed on test animals and still subject to interpretation of biological similarities.
Nano3D Biosciences has also been able to create breast cancer tumors within a 24-hour period, control the density and composition of that tumor and test the efficiencies of multiple drugs within that tumor. This eliminates human and animal testing protocols and works to accelerate drug testing and discovery.

